A biosimilar product (biosimilar) is highly similar to, and has no clinically meaningful differences from, an FDA-approved reference product. Biosimilars may spur competition...
A biosimilar product (biosimilar) is highly similar to, and has no clinically meaningful differences from, an FDA-approved reference product. Biosimilars may spur competition and result in additional safe, effective, and affordable biological product (biologic) treatments. Recognizing the potential cost-savings associated with the approval of more biosimilars, FDA and other stakeholders are working to foster more efficient biosimilar development.
Streamlining the clinical studies (studies aimed to demonstrate no clinically meaningful differences exist between a biosimilar and its reference product) can be an opportunity to speed up product development. To date, most biosimilar approvals have included pharmacokinetic (PK) similarity data (data on how the body absorbs, distributes, metabolizes, and excretes a product) and a comparative clinical study, a study whereby investigators compare the biosimilar and reference product using clinical endpoints in a patient population. A clinical endpoint study is generally time-consuming, costly, and needs a large number of participants.
A more streamlined approach may be to submit PK and pharmacodynamic (PD) similarity data (data on the relationship between drug exposure, such as dosage levels, and the body’s subsequent response) alongside comparative safety and immunogenicity data. Investigators can collect PK and PD data from shorter, less costly studies, often enrolling healthy participants.
PD similarity studies rely on PD biomarkers, which are biological molecules that signal a normal or abnormal process. PD biomarkers can demonstrate a drug’s pharmacological (or treatment-related) effect.
FDA’s Research in Biomarkers in Four Classes of Biologics
As outlined in the 2018 Biosimilars Action Plan, FDA has been conducting applied research on PD biomarkers to help facilitate biosimilar development. This research includes clinical pharmacology studies in which participants receive varying doses of a biologic and investigators determine the biomarkers’ response. A relationship between the dose and biomarker response may indicate that the biomarker is a candidate for a PD similarity study.
FDA and outside researchers have worked to identify and evaluate biomarkers in placebo-controlled studies using advanced technologies and simulations. Some of these studies were published in the January 2023 issue of Clinical Pharmacology and Therapeutics and described below 1.
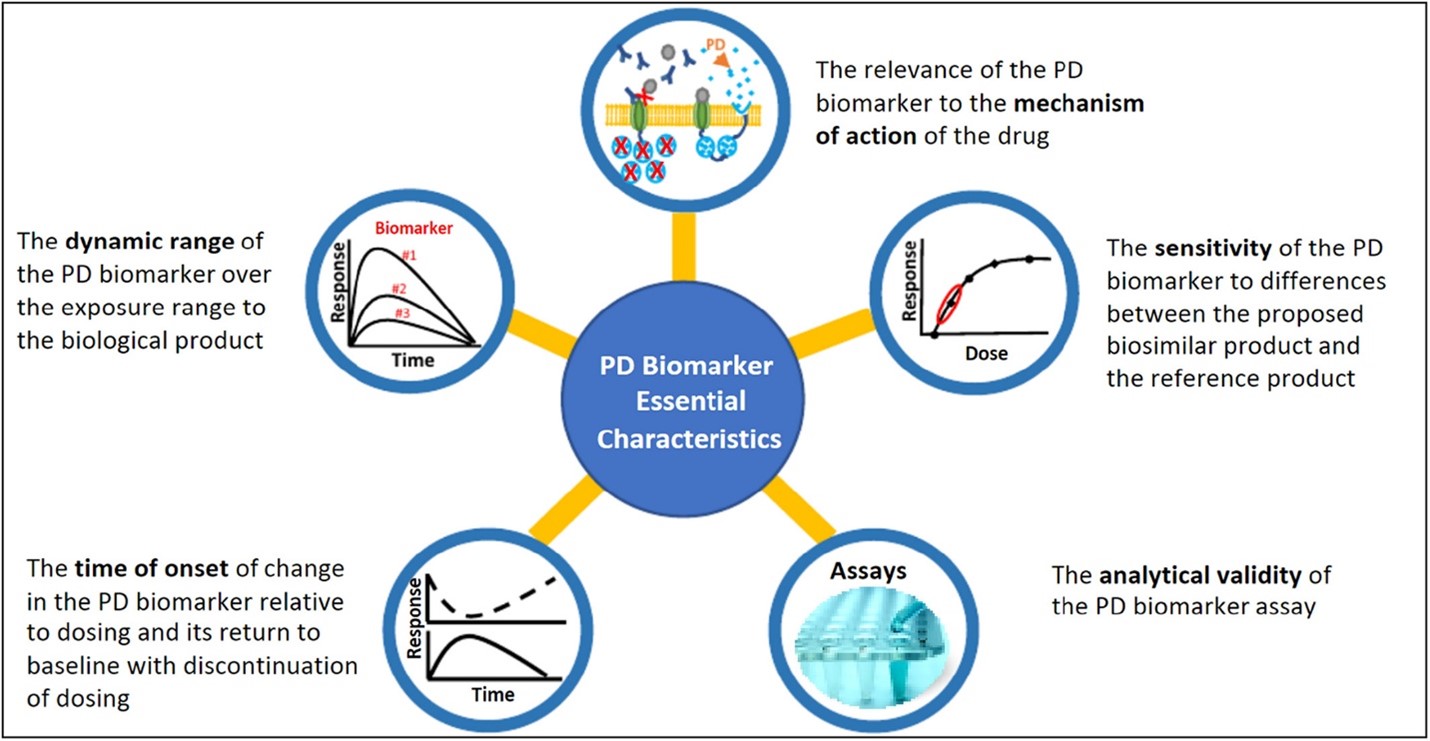
Figure 1: FDA has described five essential characteristics 2, 3 of a PD biomarker for biosimilars to help sponsors planning to use PD biomarkers in biosimilar development.
Figure published in: Strauss DG, Wang YM, Florian J, et al. Pharmacodynamic Biomarkers Evidentiary Considerations for Biosimilar Development and Approval. Clin Pharmacol Ther. 2023, 55-61. doi.org/10.1002/cpt.2761.
PCSK9 Inhibitors (Cholesterol Medications)
FDA has approved two cholesterol-lowering drugs in a class of biologics known as PCSK9 inhibitors: alirocumab and evolocumab. PCSK9 inhibitors block a protein called PCSK9 that prevents the body from getting rid of excess cholesterol.
Researchers conducted a study in which 72 healthy participants received a dose of evolocumab (21, 35, 70, or 140 mg), alirocumab (15, 25, 50, or 100 mg), or a placebo. 4 Investigators examined two biomarkers: low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B (apoB) in serum.
The primary endpoints were area under the effect curve (AUEC), which is a measure of the biomarker response over time, and maximum change from baseline of LDL-C and apoB in serum.
The study results showed clear dose–response for alirocumab and evolocumab on the PD biomarkers, meaning that there was a larger change in LDL-C and apoB from baseline as doses increased. However, for apoB, there was higher response variability among research participants. LDL-C and apoB may be suitable PD biomarkers in PD similarity studies of alirocumab and evolocumab.
Figure 2: Example temporal and dose—response data for a PD biomarker to inform identification of a sensitive dose. PD biomarkers with a large dynamic range over the range of drug concentrations observed in PK evaluation allow for evaluation of the dose–response relationship to inform the PD similarity study dose selection.
Figure published in: Strauss DG, Wang YM, Florian J, et al. Pharmacodynamic Biomarkers Evidentiary Considerations for Biosimilar Development and Approval. Clin Pharmacol Ther. 2023, 55-61. doi.org/10.1002/cpt.2761.
IL-5 Antagonists (Asthma Medications)
Anti-interleukin-5 (IL-5) is a class of biologics approved to treat severe eosinophilic asthma, a disorder characterized by too many eosinophils (a type of white blood cells). IL-5 is the main cytokine (a type of protein) that helps activate eosinophils, which in turn cause airway inflammation. IL-5 antagonists block IL-5 from activating eosinophils. Within the IL-5 class of asthma medications, there are three approved biologics: benralizumab, mepolizumab, and reslizumab.
In this study, 72 healthy participants received either placebo or a dose below the therapeutic dose of the IL-5 inhibitors mepolizumab (3–24 mg) or reslizumab (0.1–0.8 mg/kg). 5 Circulating eosinophils were the PD biomarker under study.
The primary endpoints were the maximum change from baseline and the AUEC. No dose–response relationship for eosinophil counts was observed due to high data variability among research participants, although the highest dose of mepolizumab (24 mg) and reslizumab (0.8 mg/kg) showed clear effects. Because of the high variability, researchers did not perform the planned dose–response analysis.
As such, researchers used existing models and simulations to explore doses of IL-5 antagonists up to the therapeutic dose and the effect on circulating eosinophils. Results showed that higher doses, such as the therapeutic doses, may be adequately sensitive for evaluating differences between products with circulating eosinophils as a PD biomarker in a PD similarity study.
Proteomics to Identify IFNβ-1a Biomarkers (Multiple Sclerosis Drugs)
In this study, researchers examined how proteomics — the large-scale study of proteomes, or protein sets — can identify PD biomarkers for interferon-beta 1a biologics (IFNβ-1a) or pegylated-IFNβ-1a (pegIFNβ-1a) biologics. 6 These products are used to treat multiple sclerosis, but scientists do not fully understand their mechanism of action (how they work). Experts also are not sure of the relevant PD biomarkers, although there are potential reported candidates.
To better understand the issue, researchers conducted a study and used proteomics to evaluate circulating biomarkers, assessing more than 7,000 proteins from plasma samples in search of potential PD biomarkers.
In the study, 84 healthy participants received a therapeutic dose of either interferon-beta 1a (IFNβ-1a) biologics, pegylated-IFNβ-1a (pegIFNβ-1a) biologics, or a placebo. In the initial analysis, only data from the 36 participants receiving placebo or the highest dose (30 µg IFNβ-1a or 125 µg pegIFNβ-1a) were evaluated. Researchers collected blood samples at baseline and several times thereafter and used a proteomics assay (SOMAscan assay version 4.1) to assess the proteins.
Researchers found 248 (IFNβ-1a) and 528 (pegIFNβ-1a) different proteins with different expressions between the treatment and placebo groups. Of these, researchers prioritized 31 proteins with the greatest differences from placebo, including eight proteins with a greater than four-fold maximal change from baseline. Researchers identified previously reported candidates as well as many new ones.
The study demonstrates how proteomics can help identify potential PD biomarkers that investigators can use in clinical pharmacology studies to support biosimilar development, especially when the products have complex mechanisms of action or few identified PD biomarkers.
Model-Based Approach for Dose Selection of Pegfilgrastim (Supportive Care Medications for Cancer)
Pegfilgrastim is used to treat neutropenia (low white blood cells) caused by anti-cancer medications.
Pegfilgrastim has several approved biosimilars in which a PK and PD approach, using the absolute neutrophil count (ANC) biomarker, has been used to support biosimilar approvals. Most programs used 6 mg for their PK and PD similarity studies while some programs used 2 mg. Pegfilgrastim has non-linear PK, which means that the medication’s “clearance” from the body changes with the dose. This can complicate determining an appropriate dosage to use for PK and PD similarity studies.
In this study, researchers used model-informed drug development (MIDD) approaches to help select pegfilgrastim doses for PK and PD similarity studies. MIDD approaches use quantitative methods and data sources to inform drug development and regulatory decision-making. For the study, researchers adapted a published model using data from two phase I studies, in which healthy subjects received either a single dose of 30, 60, or 100 μg/kg pegfilgrastim. Investigators performed simulations at two doses (2 mg and 6 mg).
Simulation analyses demonstrated that a 6 mg pegfilgrastim dose is appropriate for detecting differences between a proposed pegfilgrastim biosimilar and the reference product in PK and PD similarity studies. 7 This study showed that simulations can support dose selection for biosimilars with nonlinear PK.
Overall, the FDA research featured in this Spotlight has shed new insights on analysis of PD biomarker data, increased our understanding of bioanalytical considerations when analyzing PK and PD endpoints, and demonstrated the use of novel “omics” methods for identifying potential PD biomarkers for biosimilar development.
1. Innovations in Biosimilars. Clin Pharmacol Ther. 2023 Jan. 1-195. doi.org/10.1002/cpt.2653
2. US Food and Drug Administration. FDA guidance: scientific considerations in demonstrating biosimilarity to a reference product (2015). Accessed March 24, 2023.
3. US Food and Drug Administration. FDA guidance: clinical pharmacology data to support a demonstration of biosimilarity to a reference product (2016). March 24, 2023.
4. Sheikhy M, Schrieber SJ, Sun Q, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development – (I) a randomized trial with PCSK9 inhibitors. Clin Pharmacol Ther. 2023 Jan. 71-79. doi.org/10.1002/cpt.2769.
5. Gershuny V, Sun Q, Schrieber SJ, et al. Considerations for use of pharmacodynamic biomarkers to support biosimilar development – (II) a randomized trial with IL-5 antagonists. Clin Pharmacol Ther. 2023 Jan. 80-89. doi.org/10.1002/cpt.2760
6. Hyland, PL, Chekka LMS, Samarth DP, et al. Evaluating the utility of proteomics for the identification of circulating pharmacodynamic biomarkers of IFNβ-1a biologics. Clin Pharmacol Ther. 2023 Jan. 98-107. doi.org/10.1002/cpt.2778
7. Li F, Sun Q, Du S, et al. Model-Based Approach to Selecting Pegfilgrastim Dose for Pharmacokinetic and Pharmacodynamic Similarity Studies in Biosimilar Development. Clin Pharmacol Ther. 2023 Jan. 62-70.